Binding-Pocket Interactions of Four EGFR Inhibitors
For this notebook, we use mdciao to visualize the binding-pocket interactions of four Epidermal Growth Factor Receptor (EGFR) inhibitors. EGFR is an important drug target with implications in cancer and inflammation (Wikipedia). It is a transmembrane protein with an extracellular receptor domain and an intracellular kinase domain.
The molecular dynamics (MD) data used here was generated by slightly modifying the notebook
which is part of the impressive TeachOpenCADD collection, made available as teaching platform for computer-aided drug design by the Volkamer Lab at Saarland University, Saarbrücken.
The four inhibitors and structures are chosen from the following RCSB entries:
*The crystal structure of EGFR T790M/C797S with the inhibitor HCD2892 (PDB ID 7VRE)*
*EGFR kinase domain complexed with compound 20a (PDB ID 3W32)*
*Crystal Structure of EGFR(L858R/T790M/C797S) in complex with CH7233163 (PDB ID 6LUB)*
Please see the references at the bottom of the notebook for more information.
[1]:
import mdciao
import os
import matplotlib
import nglview
from glob import glob
Consensus labeler object for KLIFS nomenclature
Since it will be used more than once, it is better to have it instantiated only once and reused many times. The only thing we need is the UniProt Accession Code of the EGFR, P00533.
[2]:
KLIFS = mdciao.nomenclature.LabelerKLIFS("UniProtAC:P00533")
No local file ./KLIFS_UniProtAC:P00533.xlsx found, checking online in
https://klifs.net/api/kinase_ID?kinase_name=UniProtAC:P00533 ...https://klifs.net/api/structures_list?kinase_ID=406
done!
Please cite the following reference to the KLIF structural database:
* Kanev et al, (2021) KLIFS: an overhaul after the first 5 years of supporting kinase research
Nucleic Acids Research 49, D562--D569
https://doi.org/10.1093/NAR/GKAA895
For more information, call mdciao.nomenclature.references()
/home/perezheg/miniconda3/lib/python3.11/site-packages/mdtraj/formats/pdb/pdbfile.py:208: UserWarning: Unlikely unit cell vectors detected in PDB file likely resulting from a dummy CRYST1 record. Discarding unit cell vectors.
warnings.warn(
Download example data
[3]:
if not os.path.exists("example_kinases"):
mdciao.examples.fetch_example_data("EGFR");
Unzipping to 'example_kinases'
Guess molecular fragments
[4]:
for pdb in sorted(glob("example_kinases/*.pdb")):
print(pdb)
mdciao.fragments.get_fragments(pdb)
print()
example_kinases/topology.3POZ.pdb
Auto-detected fragments with method 'lig_resSeq+'
fragment 0 with 317 AAs GLN701 ( 0) - LEU1017 (316 ) (0)
fragment 1 with 1 AAs 03P1 ( 317) - 03P1 (317 ) (1)
example_kinases/topology.3W32.pdb
Auto-detected fragments with method 'lig_resSeq+'
fragment 0 with 317 AAs GLN701 ( 0) - LEU1017 (316 ) (0)
fragment 1 with 1 AAs W321 ( 317) - W321 (317 ) (1)
example_kinases/topology.6LUB.pdb
Auto-detected fragments with method 'lig_resSeq+'
fragment 0 with 323 AAs GLY696 ( 0) - ILE1018 (322 ) (0)
fragment 1 with 1 AAs EUX1 ( 323) - EUX1 (323 ) (1)
example_kinases/topology.7VRE.pdb
Auto-detected fragments with method 'lig_resSeq+'
fragment 0 with 323 AAs GLY696 ( 0) - ILE1018 (322 ) (0)
fragment 1 with 1 AAs 7VH1 ( 323) - 7VH1 (323 ) (1)
All three setups share the equivalent topology of kinase (fragment 0) and ligand (fragment 1):
from PDB ID
3POZligand03P1from PDB ID
3W32ligandW321from PDB ID
6LUBligandEUX1from PDB ID
7VREligand7VH1
For labelling purposes, create a mapping between PDB IDs and ligand names:
[5]:
pdb2lig = {"3POZ" : "03P1",
"3W32" : "W321",
"6LUB" : "EUX1",
"7VRE" : "7VH1"
}
Compute the ligand-kinase interactions for the four inhibitors
[6]:
binding_pocket = {}
for pdb in sorted(glob("example_kinases/*.pdb")):
key = os.path.basename(pdb).split(".")[1]
key="%s@%s"%(pdb2lig[key], key)
xtc = pdb.replace(".pdb",".xtc").replace("topology","trajectory")
binding_pocket[key]=mdciao.cli.interface(xtc,
pdb,
fragment_names=["EGFR", "ligand"],
KLIFS_string=KLIFS,
ctc_control=1.0,
interface_selection_1=[0],
interface_selection_2=[1],
accept_guess=True, figures=False, no_disk=True)
Will compute contact frequencies for trajectories:
example_kinases/trajectory.3POZ.xtc
with a stride of 1 frames
Using method 'lig_resSeq+' these fragments were found
fragment EGFR with 317 AAs GLN701 ( 0) - LEU1017 (316 ) (EGFR)
fragment ligand with 1 AAs 03P1 ( 317) - 03P1 (317 ) (ligand)
The KLIFS-labels align best with fragments: [0] (first-last: GLN701-LEU1017).
Mapping the KLIFS fragments onto your topology:
I with 3 AAs LYS716@I.1 ( 15) - LEU718@I.3 (17 ) (I)
g.l with 6 AAs GLY719@g.l.4 ( 18) - GLY724@g.l.9 (23 ) (g.l)
II with 4 AAs THR725@II.10 ( 24) - LYS728@II.13 (27 ) (II)
III with 6 AAs VAL742@III.14 ( 41) - LEU747@III.19 (46 ) (III)
αC with 11 AAs GLU758@αC.20 ( 57) - SER768@αC.30 (67 ) (αC)
b.l with 7 AAs VAL769@b.l.31 ( 68) - ARG776@b.l.37 (75 ) (b.l) resSeq jumps
IV with 4 AAs LEU777@IV.38 ( 76) - ILE780@IV.41 (79 ) (IV)
V with 3 AAs GLN787@V.42 ( 86) - ILE789@V.44 (88 ) (V)
GK with 1 AAs THR790@GK.45 ( 89) - THR790@GK.45 (89 ) (GK)
hinge with 3 AAs GLN791@hinge.46 ( 90) - MET793@hinge.48 (92 ) (hinge)
linker with 4 AAs PRO794@linker.49 ( 93) - CYS797@linker.52 (96 ) (linker)
αD with 7 AAs LEU798@αD.53 ( 97) - GLU804@αD.59 (103 ) (αD)
αE with 5 AAs TYR827@αE.60 ( 126) - ARG831@αE.64 (130 ) (αE)
VI with 3 AAs ARG832@VI.65 ( 131) - VAL834@VI.67 (133 ) (VI)
c.l with 8 AAs HIS835@c.l.68 ( 134) - ASN842@c.l.75 (141 ) (c.l)
VII with 3 AAs VAL843@VII.76 ( 142) - VAL845@VII.78 (144 ) (VII)
VIII with 1 AAs ILE853@VIII.79 ( 152) - ILE853@VIII.79 (152 ) (VIII)
xDFG with 4 AAs THR854@xDFG.80 ( 153) - GLY857@xDFG.83 (156 ) (xDFG)
a.l with 2 AAs LEU858@a.l.84 ( 157) - ALA859@a.l.85 (158 ) (a.l)
Select group 1: 0
Select group 2: 1
Will look for contacts in the interface between fragments
0
and
1.
Performing a first pass on the 317 group_1-group_2 residue pairs to compute lower bounds on residue-residue distances via residue-COM distances.
Reduced to only 220 (from 317) residue pairs for the computation of actual residue-residue distances:
The following 30 contacts capture 25.13 (~99%) of the total frequency 25.28 (over 36 contacts with nonzero frequency at 4.50 Angstrom).
As orientation value, the first 25 ctcs already capture 90.0% of 25.28.
The 25-th contact has a frequency of 0.55.
freq label residues fragments sum
1 1.00 L792@hinge.47 - 03P1@ligand 91 - 317 0 - 1 1.00
2 1.00 M793@hinge.48 - 03P1@ligand 92 - 317 0 - 1 2.00
3 1.00 L777@IV.38 - 03P1@ligand 76 - 317 0 - 1 3.00
4 1.00 L844@VII.77 - 03P1@ligand 143 - 317 0 - 1 4.00
5 1.00 T854@xDFG.80 - 03P1@ligand 153 - 317 0 - 1 5.00
6 1.00 D855@xDFG.81 - 03P1@ligand 154 - 317 0 - 1 6.00
7 1.00 F856@xDFG.82 - 03P1@ligand 155 - 317 0 - 1 7.00
8 1.00 T790@GK.45 - 03P1@ligand 89 - 317 0 - 1 8.00
9 1.00 K745@III.17 - 03P1@ligand 44 - 317 0 - 1 9.00
10 1.00 C775@b.l.36 - 03P1@ligand 74 - 317 0 - 1 10.00
11 1.00 Q791@hinge.46 - 03P1@ligand 90 - 317 0 - 1 11.00
12 1.00 A743@III.15 - 03P1@ligand 42 - 317 0 - 1 12.00
13 1.00 L788@V.43 - 03P1@ligand 87 - 317 0 - 1 13.00
14 0.99 V726@II.11 - 03P1@ligand 25 - 317 0 - 1 13.99
15 0.99 R776@b.l.37 - 03P1@ligand 75 - 317 0 - 1 14.99
16 0.99 M766@αC.28 - 03P1@ligand 65 - 317 0 - 1 15.98
17 0.98 L718@I.3 - 03P1@ligand 17 - 317 0 - 1 16.95
18 0.91 I744@III.16 - 03P1@ligand 43 - 317 0 - 1 17.87
19 0.86 S720@g.l.5 - 03P1@ligand 19 - 317 0 - 1 18.72
20 0.85 R841@c.l.74 - 03P1@ligand 140 - 317 0 - 1 19.57
21 0.82 L858@a.l.84 - 03P1@ligand 157 - 317 0 - 1 20.39
22 0.81 G796@linker.51 - 03P1@ligand 95 - 317 0 - 1 21.20
23 0.80 F997@EGFR - 03P1@ligand 296 - 317 0 - 1 22.00
24 0.68 I789@V.44 - 03P1@ligand 88 - 317 0 - 1 22.68
25 0.55 G719@g.l.4 - 03P1@ligand 18 - 317 0 - 1 23.22
26 0.50 G721@g.l.6 - 03P1@ligand 20 - 317 0 - 1 23.72
27 0.47 C797@linker.52 - 03P1@ligand 96 - 317 0 - 1 24.19
28 0.44 N842@c.l.75 - 03P1@ligand 141 - 317 0 - 1 24.63
29 0.36 I853@VIII.79 - 03P1@ligand 152 - 317 0 - 1 24.99
30 0.14 D800@αD.55 - 03P1@ligand 99 - 317 0 - 1 25.13
label freq
1 L792@hinge.47 1.00
2 M793@hinge.48 1.00
3 L777@IV.38 1.00
4 L844@VII.77 1.00
5 T854@xDFG.80 1.00
6 D855@xDFG.81 1.00
7 F856@xDFG.82 1.00
8 T790@GK.45 1.00
9 K745@III.17 1.00
10 C775@b.l.36 1.00
11 Q791@hinge.46 1.00
12 A743@III.15 1.00
13 L788@V.43 1.00
14 V726@II.11 0.99
15 R776@b.l.37 0.99
16 M766@αC.28 0.99
17 L718@I.3 0.98
18 I744@III.16 0.91
19 S720@g.l.5 0.86
20 R841@c.l.74 0.85
21 L858@a.l.84 0.82
22 G796@linker.51 0.81
23 F997@EGFR 0.80
24 I789@V.44 0.68
25 G719@g.l.4 0.55
26 G721@g.l.6 0.50
27 C797@linker.52 0.47
28 N842@c.l.75 0.44
29 I853@VIII.79 0.36
30 D800@αD.55 0.14
label freq
1 03P1@ligand 25.13
Will compute contact frequencies for trajectories:
example_kinases/trajectory.3W32.xtc
with a stride of 1 frames
Using method 'lig_resSeq+' these fragments were found
fragment EGFR with 317 AAs GLN701 ( 0) - LEU1017 (316 ) (EGFR)
fragment ligand with 1 AAs W321 ( 317) - W321 (317 ) (ligand)
The KLIFS-labels align best with fragments: [0] (first-last: GLN701-LEU1017).
Mapping the KLIFS fragments onto your topology:
I with 3 AAs LYS716@I.1 ( 15) - LEU718@I.3 (17 ) (I)
g.l with 6 AAs GLY719@g.l.4 ( 18) - GLY724@g.l.9 (23 ) (g.l)
II with 4 AAs THR725@II.10 ( 24) - LYS728@II.13 (27 ) (II)
III with 6 AAs VAL742@III.14 ( 41) - LEU747@III.19 (46 ) (III)
αC with 11 AAs GLU758@αC.20 ( 57) - SER768@αC.30 (67 ) (αC)
b.l with 7 AAs VAL769@b.l.31 ( 68) - ARG776@b.l.37 (75 ) (b.l) resSeq jumps
IV with 4 AAs LEU777@IV.38 ( 76) - ILE780@IV.41 (79 ) (IV)
V with 3 AAs GLN787@V.42 ( 86) - ILE789@V.44 (88 ) (V)
GK with 1 AAs THR790@GK.45 ( 89) - THR790@GK.45 (89 ) (GK)
hinge with 3 AAs GLN791@hinge.46 ( 90) - MET793@hinge.48 (92 ) (hinge)
linker with 4 AAs PRO794@linker.49 ( 93) - CYS797@linker.52 (96 ) (linker)
αD with 7 AAs LEU798@αD.53 ( 97) - GLU804@αD.59 (103 ) (αD)
αE with 5 AAs TYR827@αE.60 ( 126) - ARG831@αE.64 (130 ) (αE)
VI with 3 AAs ARG832@VI.65 ( 131) - VAL834@VI.67 (133 ) (VI)
c.l with 8 AAs HIS835@c.l.68 ( 134) - ASN842@c.l.75 (141 ) (c.l)
VII with 3 AAs VAL843@VII.76 ( 142) - VAL845@VII.78 (144 ) (VII)
VIII with 1 AAs ILE853@VIII.79 ( 152) - ILE853@VIII.79 (152 ) (VIII)
xDFG with 4 AAs THR854@xDFG.80 ( 153) - GLY857@xDFG.83 (156 ) (xDFG)
a.l with 2 AAs LEU858@a.l.84 ( 157) - ALA859@a.l.85 (158 ) (a.l)
Select group 1: 0
Select group 2: 1
Will look for contacts in the interface between fragments
0
and
1.
Performing a first pass on the 317 group_1-group_2 residue pairs to compute lower bounds on residue-residue distances via residue-COM distances.
Reduced to only 200 (from 317) residue pairs for the computation of actual residue-residue distances:
The following 31 contacts capture 26.59 (~100%) of the total frequency 26.67 (over 34 contacts with nonzero frequency at 4.50 Angstrom).
As orientation value, the first 25 ctcs already capture 90.0% of 26.67.
The 25-th contact has a frequency of 0.81.
freq label residues fragments sum
1 1.00 T790@GK.45 - W321@ligand 89 - 317 0 - 1 1.00
2 1.00 L792@hinge.47 - W321@ligand 91 - 317 0 - 1 2.00
3 1.00 C775@b.l.36 - W321@ligand 74 - 317 0 - 1 3.00
4 1.00 L788@V.43 - W321@ligand 87 - 317 0 - 1 4.00
5 1.00 T854@xDFG.80 - W321@ligand 153 - 317 0 - 1 5.00
6 1.00 D855@xDFG.81 - W321@ligand 154 - 317 0 - 1 6.00
7 1.00 F856@xDFG.82 - W321@ligand 155 - 317 0 - 1 7.00
8 1.00 K745@III.17 - W321@ligand 44 - 317 0 - 1 8.00
9 1.00 L777@IV.38 - W321@ligand 76 - 317 0 - 1 9.00
10 1.00 Q791@hinge.46 - W321@ligand 90 - 317 0 - 1 10.00
11 1.00 A743@III.15 - W321@ligand 42 - 317 0 - 1 11.00
12 1.00 M793@hinge.48 - W321@ligand 92 - 317 0 - 1 12.00
13 1.00 V726@II.11 - W321@ligand 25 - 317 0 - 1 13.00
14 1.00 R776@b.l.37 - W321@ligand 75 - 317 0 - 1 14.00
15 1.00 M766@αC.28 - W321@ligand 65 - 317 0 - 1 15.00
16 1.00 L844@VII.77 - W321@ligand 143 - 317 0 - 1 16.00
17 1.00 G719@g.l.4 - W321@ligand 18 - 317 0 - 1 16.99
18 0.99 L718@I.3 - W321@ligand 17 - 317 0 - 1 17.99
19 0.98 G796@linker.51 - W321@ligand 95 - 317 0 - 1 18.97
20 0.97 S720@g.l.5 - W321@ligand 19 - 317 0 - 1 19.94
21 0.88 I744@III.16 - W321@ligand 43 - 317 0 - 1 20.82
22 0.86 I789@V.44 - W321@ligand 88 - 317 0 - 1 21.68
23 0.83 L858@a.l.84 - W321@ligand 157 - 317 0 - 1 22.51
24 0.82 C797@linker.52 - W321@ligand 96 - 317 0 - 1 23.33
25 0.81 L1001@EGFR - W321@ligand 300 - 317 0 - 1 24.14
26 0.74 G721@g.l.6 - W321@ligand 20 - 317 0 - 1 24.89
27 0.54 I853@VIII.79 - W321@ligand 152 - 317 0 - 1 25.42
28 0.47 R841@c.l.74 - W321@ligand 140 - 317 0 - 1 25.90
29 0.24 F997@EGFR - W321@ligand 296 - 317 0 - 1 26.14
30 0.23 D800@αD.55 - W321@ligand 99 - 317 0 - 1 26.38
31 0.22 G724@g.l.9 - W321@ligand 23 - 317 0 - 1 26.59
label freq
1 T790@GK.45 1.00
2 L792@hinge.47 1.00
3 C775@b.l.36 1.00
4 L788@V.43 1.00
5 T854@xDFG.80 1.00
6 D855@xDFG.81 1.00
7 F856@xDFG.82 1.00
8 K745@III.17 1.00
9 L777@IV.38 1.00
10 Q791@hinge.46 1.00
11 A743@III.15 1.00
12 M793@hinge.48 1.00
13 V726@II.11 1.00
14 R776@b.l.37 1.00
15 M766@αC.28 1.00
16 L844@VII.77 1.00
17 G719@g.l.4 1.00
18 L718@I.3 0.99
19 G796@linker.51 0.98
20 S720@g.l.5 0.97
21 I744@III.16 0.88
22 I789@V.44 0.86
23 L858@a.l.84 0.83
24 C797@linker.52 0.82
25 L1001@EGFR 0.81
26 G721@g.l.6 0.74
27 I853@VIII.79 0.54
28 R841@c.l.74 0.47
29 F997@EGFR 0.24
30 D800@αD.55 0.23
31 G724@g.l.9 0.22
label freq
1 W321@ligand 26.59
Will compute contact frequencies for trajectories:
example_kinases/trajectory.6LUB.xtc
with a stride of 1 frames
Using method 'lig_resSeq+' these fragments were found
fragment EGFR with 323 AAs GLY696 ( 0) - ILE1018 (322 ) (EGFR)
fragment ligand with 1 AAs EUX1 ( 323) - EUX1 (323 ) (ligand)
The KLIFS-labels align best with fragments: [0] (first-last: GLY696-ILE1018).
Mapping the KLIFS fragments onto your topology:
I with 3 AAs LYS716@I.1 ( 20) - LEU718@I.3 (22 ) (I)
g.l with 6 AAs GLY719@g.l.4 ( 23) - GLY724@g.l.9 (28 ) (g.l)
II with 4 AAs THR725@II.10 ( 29) - LYS728@II.13 (32 ) (II)
III with 6 AAs VAL742@III.14 ( 46) - LEU747@III.19 (51 ) (III)
αC with 11 AAs GLU758@αC.20 ( 62) - SER768@αC.30 (72 ) (αC)
b.l with 7 AAs VAL769@b.l.31 ( 73) - ARG776@b.l.37 (80 ) (b.l) resSeq jumps
IV with 4 AAs LEU777@IV.38 ( 81) - ILE780@IV.41 (84 ) (IV)
V with 3 AAs GLN787@V.42 ( 91) - ILE789@V.44 (93 ) (V)
GK with 1 AAs MET790@GK.45 ( 94) - MET790@GK.45 (94 ) (GK)
hinge with 3 AAs GLN791@hinge.46 ( 95) - MET793@hinge.48 (97 ) (hinge)
linker with 4 AAs PRO794@linker.49 ( 98) - SER797@linker.52 (101 ) (linker)
αD with 7 AAs LEU798@αD.53 ( 102) - GLU804@αD.59 (108 ) (αD)
αE with 5 AAs TYR827@αE.60 ( 131) - ARG831@αE.64 (135 ) (αE)
VI with 3 AAs ARG832@VI.65 ( 136) - VAL834@VI.67 (138 ) (VI)
c.l with 8 AAs HIS835@c.l.68 ( 139) - ASN842@c.l.75 (146 ) (c.l)
VII with 3 AAs VAL843@VII.76 ( 147) - VAL845@VII.78 (149 ) (VII)
VIII with 1 AAs ILE853@VIII.79 ( 157) - ILE853@VIII.79 (157 ) (VIII)
xDFG with 4 AAs THR854@xDFG.80 ( 158) - GLY857@xDFG.83 (161 ) (xDFG)
a.l with 2 AAs ARG858@a.l.84 ( 162) - ALA859@a.l.85 (163 ) (a.l)
Select group 1: 0
Select group 2: 1
Will look for contacts in the interface between fragments
0
and
1.
Performing a first pass on the 323 group_1-group_2 residue pairs to compute lower bounds on residue-residue distances via residue-COM distances.
Reduced to only 190 (from 323) residue pairs for the computation of actual residue-residue distances:
The following 25 contacts capture 20.50 (~98%) of the total frequency 20.83 (over 39 contacts with nonzero frequency at 4.50 Angstrom).
As orientation value, the first 20 ctcs already capture 90.0% of 20.83.
The 20-th contact has a frequency of 0.64.
freq label residues fragments sum
1 1.00 G796@linker.51 - EUX1@ligand 100 - 323 0 - 1 1.00
2 1.00 V726@II.11 - EUX1@ligand 30 - 323 0 - 1 2.00
3 1.00 P794@linker.49 - EUX1@ligand 98 - 323 0 - 1 3.00
4 1.00 L718@I.3 - EUX1@ligand 22 - 323 0 - 1 4.00
5 1.00 M793@hinge.48 - EUX1@ligand 97 - 323 0 - 1 5.00
6 1.00 L844@VII.77 - EUX1@ligand 148 - 323 0 - 1 6.00
7 1.00 L792@hinge.47 - EUX1@ligand 96 - 323 0 - 1 7.00
8 1.00 Q791@hinge.46 - EUX1@ligand 95 - 323 0 - 1 8.00
9 1.00 M790@GK.45 - EUX1@ligand 94 - 323 0 - 1 9.00
10 1.00 A743@III.15 - EUX1@ligand 47 - 323 0 - 1 10.00
11 0.98 G724@g.l.9 - EUX1@ligand 28 - 323 0 - 1 10.97
12 0.97 T854@xDFG.80 - EUX1@ligand 158 - 323 0 - 1 11.94
13 0.96 K745@III.17 - EUX1@ligand 49 - 323 0 - 1 12.90
14 0.95 S797@linker.52 - EUX1@ligand 101 - 323 0 - 1 13.85
15 0.92 G721@g.l.6 - EUX1@ligand 25 - 323 0 - 1 14.78
16 0.92 K728@II.13 - EUX1@ligand 32 - 323 0 - 1 15.70
17 0.92 G719@g.l.4 - EUX1@ligand 23 - 323 0 - 1 16.61
18 0.88 T725@II.10 - EUX1@ligand 29 - 323 0 - 1 17.49
19 0.82 C775@b.l.36 - EUX1@ligand 79 - 323 0 - 1 18.31
20 0.64 F795@linker.50 - EUX1@ligand 99 - 323 0 - 1 18.95
21 0.48 D855@xDFG.81 - EUX1@ligand 159 - 323 0 - 1 19.43
22 0.43 S720@g.l.5 - EUX1@ligand 24 - 323 0 - 1 19.86
23 0.32 D800@αD.55 - EUX1@ligand 104 - 323 0 - 1 20.17
24 0.17 R841@c.l.74 - EUX1@ligand 145 - 323 0 - 1 20.34
25 0.15 L1001@EGFR - EUX1@ligand 305 - 323 0 - 1 20.50
label freq
1 G796@linker.51 1.00
2 V726@II.11 1.00
3 P794@linker.49 1.00
4 L718@I.3 1.00
5 M793@hinge.48 1.00
6 L844@VII.77 1.00
7 L792@hinge.47 1.00
8 Q791@hinge.46 1.00
9 M790@GK.45 1.00
10 A743@III.15 1.00
11 G724@g.l.9 0.98
12 T854@xDFG.80 0.97
13 K745@III.17 0.96
14 S797@linker.52 0.95
15 G721@g.l.6 0.92
16 K728@II.13 0.92
17 G719@g.l.4 0.92
18 T725@II.10 0.88
19 C775@b.l.36 0.82
20 F795@linker.50 0.64
21 D855@xDFG.81 0.48
22 S720@g.l.5 0.43
23 D800@αD.55 0.32
24 R841@c.l.74 0.17
25 L1001@EGFR 0.15
label freq
1 EUX1@ligand 20.5
Will compute contact frequencies for trajectories:
example_kinases/trajectory.7VRE.xtc
with a stride of 1 frames
Using method 'lig_resSeq+' these fragments were found
fragment EGFR with 323 AAs GLY696 ( 0) - ILE1018 (322 ) (EGFR)
fragment ligand with 1 AAs 7VH1 ( 323) - 7VH1 (323 ) (ligand)
The KLIFS-labels align best with fragments: [0] (first-last: GLY696-ILE1018).
Mapping the KLIFS fragments onto your topology:
I with 3 AAs LYS716@I.1 ( 20) - LEU718@I.3 (22 ) (I)
g.l with 6 AAs GLY719@g.l.4 ( 23) - GLY724@g.l.9 (28 ) (g.l)
II with 4 AAs THR725@II.10 ( 29) - LYS728@II.13 (32 ) (II)
III with 6 AAs VAL742@III.14 ( 46) - LEU747@III.19 (51 ) (III)
αC with 11 AAs GLU758@αC.20 ( 62) - SER768@αC.30 (72 ) (αC)
b.l with 7 AAs VAL769@b.l.31 ( 73) - ARG776@b.l.37 (80 ) (b.l) resSeq jumps
IV with 4 AAs LEU777@IV.38 ( 81) - ILE780@IV.41 (84 ) (IV)
V with 3 AAs GLN787@V.42 ( 91) - ILE789@V.44 (93 ) (V)
GK with 1 AAs MET790@GK.45 ( 94) - MET790@GK.45 (94 ) (GK)
hinge with 3 AAs GLN791@hinge.46 ( 95) - MET793@hinge.48 (97 ) (hinge)
linker with 4 AAs PRO794@linker.49 ( 98) - SER797@linker.52 (101 ) (linker)
αD with 7 AAs LEU798@αD.53 ( 102) - GLU804@αD.59 (108 ) (αD)
αE with 5 AAs TYR827@αE.60 ( 131) - ARG831@αE.64 (135 ) (αE)
VI with 3 AAs ARG832@VI.65 ( 136) - VAL834@VI.67 (138 ) (VI)
c.l with 8 AAs HIS835@c.l.68 ( 139) - ASN842@c.l.75 (146 ) (c.l)
VII with 3 AAs VAL843@VII.76 ( 147) - VAL845@VII.78 (149 ) (VII)
VIII with 1 AAs ILE853@VIII.79 ( 157) - ILE853@VIII.79 (157 ) (VIII)
xDFG with 4 AAs THR854@xDFG.80 ( 158) - GLY857@xDFG.83 (161 ) (xDFG)
a.l with 2 AAs LEU858@a.l.84 ( 162) - ALA859@a.l.85 (163 ) (a.l)
Select group 1: 0
Select group 2: 1
Will look for contacts in the interface between fragments
0
and
1.
Performing a first pass on the 323 group_1-group_2 residue pairs to compute lower bounds on residue-residue distances via residue-COM distances.
Reduced to only 212 (from 323) residue pairs for the computation of actual residue-residue distances:
The following 23 contacts capture 16.70 (~99%) of the total frequency 16.79 (over 30 contacts with nonzero frequency at 4.50 Angstrom).
As orientation value, the first 17 ctcs already capture 90.0% of 16.79.
The 17-th contact has a frequency of 0.53.
freq label residues fragments sum
1 1.00 L718@I.3 - 7VH1@ligand 22 - 323 0 - 1 1.00
2 1.00 M793@hinge.48 - 7VH1@ligand 97 - 323 0 - 1 2.00
3 1.00 L792@hinge.47 - 7VH1@ligand 96 - 323 0 - 1 3.00
4 1.00 V726@II.11 - 7VH1@ligand 30 - 323 0 - 1 4.00
5 1.00 A743@III.15 - 7VH1@ligand 47 - 323 0 - 1 5.00
6 1.00 P794@linker.49 - 7VH1@ligand 98 - 323 0 - 1 6.00
7 1.00 G796@linker.51 - 7VH1@ligand 100 - 323 0 - 1 6.99
8 1.00 Q791@hinge.46 - 7VH1@ligand 95 - 323 0 - 1 7.99
9 0.99 L844@VII.77 - 7VH1@ligand 148 - 323 0 - 1 8.98
10 0.96 F723@g.l.8 - 7VH1@ligand 27 - 323 0 - 1 9.93
11 0.93 G719@g.l.4 - 7VH1@ligand 23 - 323 0 - 1 10.86
12 0.90 M790@GK.45 - 7VH1@ligand 94 - 323 0 - 1 11.76
13 0.88 T854@xDFG.80 - 7VH1@ligand 158 - 323 0 - 1 12.64
14 0.79 D855@xDFG.81 - 7VH1@ligand 159 - 323 0 - 1 13.44
15 0.76 K745@III.17 - 7VH1@ligand 49 - 323 0 - 1 14.20
16 0.68 L1001@EGFR - 7VH1@ligand 305 - 323 0 - 1 14.88
17 0.53 R841@c.l.74 - 7VH1@ligand 145 - 323 0 - 1 15.41
18 0.44 F795@linker.50 - 7VH1@ligand 99 - 323 0 - 1 15.85
19 0.20 C775@b.l.36 - 7VH1@ligand 79 - 323 0 - 1 16.05
20 0.19 N842@c.l.75 - 7VH1@ligand 146 - 323 0 - 1 16.24
21 0.18 K728@II.13 - 7VH1@ligand 32 - 323 0 - 1 16.41
22 0.16 S797@linker.52 - 7VH1@ligand 101 - 323 0 - 1 16.58
23 0.12 D800@αD.55 - 7VH1@ligand 104 - 323 0 - 1 16.70
label freq
1 L718@I.3 1.00
2 M793@hinge.48 1.00
3 L792@hinge.47 1.00
4 V726@II.11 1.00
5 A743@III.15 1.00
6 P794@linker.49 1.00
7 G796@linker.51 1.00
8 Q791@hinge.46 1.00
9 L844@VII.77 0.99
10 F723@g.l.8 0.96
11 G719@g.l.4 0.93
12 M790@GK.45 0.90
13 T854@xDFG.80 0.88
14 D855@xDFG.81 0.79
15 K745@III.17 0.76
16 L1001@EGFR 0.68
17 R841@c.l.74 0.53
18 F795@linker.50 0.44
19 C775@b.l.36 0.20
20 N842@c.l.75 0.19
21 K728@II.13 0.18
22 S797@linker.52 0.16
23 D800@αD.55 0.12
label freq
1 7VH1@ligand 16.7
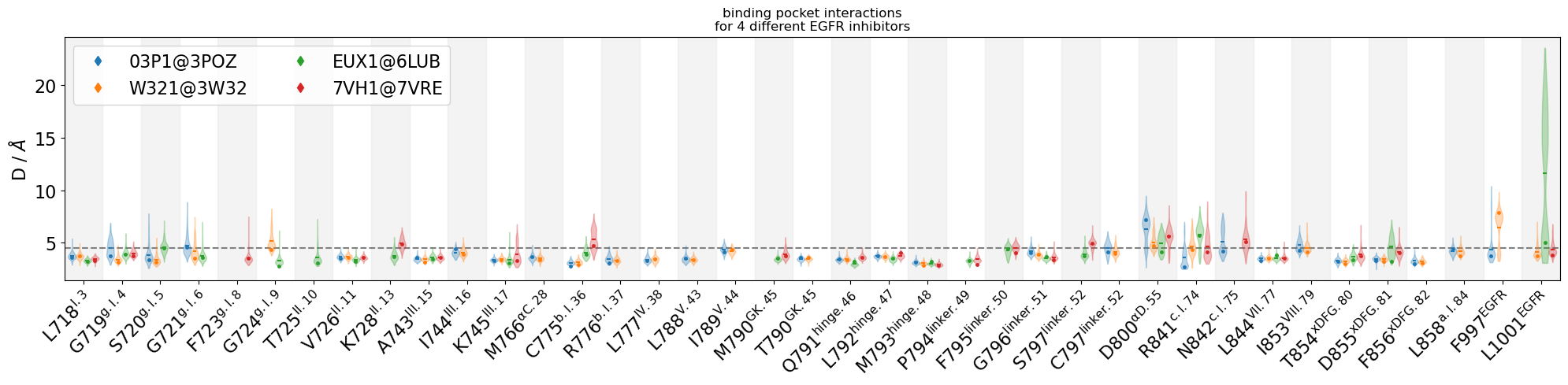
Compare interactions across the four compounds in a violinplot
Additionally, we will display representative geometries directly on the violinplots via their residue-residue distance-values. Subsequently, we will view these geometries in 3D
[7]:
colors = mdciao.plots.color_dict_guesser("tab10", binding_pocket.keys())
myfig, myax, keys, representatives = mdciao.plots.compare_violins(binding_pocket,
colors=colors,
anchor="ligand",
ctc_cutoff_Ang=4.5,
mutations_dict={
"EUX1": "ligand",
"7VH1": "ligand",
"W321": "ligand",
"03P1": "ligand"
},
defrag=None,
sort_by="residue",
legend_rows=2,
representatives=True,
figsize=(20,5)
)
myax.set_title("binding pocket interactions"
"\nfor 4 different EGFR inhibitors")
myfig.tight_layout()
#myfig.savefig("EGFR.png", bbox_inches="tight")
Returning frame 83 of traj nr. 0: example_kinases/trajectory.3POZ.xtc
Returning frame 237 of traj nr. 0: example_kinases/trajectory.3W32.xtc
Returning frame 369 of traj nr. 0: example_kinases/trajectory.6LUB.xtc
Returning frame 304 of traj nr. 0: example_kinases/trajectory.7VRE.xtc
Show the representative geometries
The object representatives is a dictionary containing the geometries behind the small dots inside the violins of the previous figure, using the repframes method. In the next cells we will first align them and then overlap them using the KLIFS nomenclature.
Superpose structures using the KLIFs alignment labels
This way, the alignment will be particularly good in the binding pocket
[8]:
KLIFS_alignment = mdciao.nomenclature.AlignerConsensus({key : KLIFS for key in binding_pocket.keys()},
tops={key : bp.top for key, bp in binding_pocket.items()})
KLIFS_alignment.AAresSeq
[8]:
| consensus | 03P1@3POZ | W321@3W32 | EUX1@6LUB | 7VH1@7VRE | |
|---|---|---|---|---|---|
| 0 | I.1 | K716 | K716 | K716 | K716 |
| 1 | I.2 | V717 | V717 | V717 | V717 |
| 2 | I.3 | L718 | L718 | L718 | L718 |
| 3 | g.l.4 | G719 | G719 | G719 | G719 |
| 4 | g.l.5 | S720 | S720 | S720 | S720 |
| ... | ... | ... | ... | ... | ... |
| 80 | xDFG.81 | D855 | D855 | D855 | D855 |
| 81 | xDFG.82 | F856 | F856 | F856 | F856 |
| 82 | xDFG.83 | G857 | G857 | G857 | G857 |
| 83 | a.l.84 | L858 | L858 | R858 | L858 |
| 84 | a.l.85 | A859 | A859 | A859 | A859 |
85 rows × 5 columns
[9]:
# We can directly get CA indices to map atoms
KLIFS_alignment.CAidxs
[9]:
| consensus | 03P1@3POZ | W321@3W32 | EUX1@6LUB | 7VH1@7VRE | |
|---|---|---|---|---|---|
| 0 | I.1 | 280 | 280 | 340 | 340 |
| 1 | I.2 | 302 | 302 | 362 | 362 |
| 2 | I.3 | 318 | 318 | 378 | 378 |
| 3 | g.l.4 | 337 | 337 | 397 | 397 |
| 4 | g.l.5 | 344 | 344 | 404 | 404 |
| ... | ... | ... | ... | ... | ... |
| 80 | xDFG.81 | 2515 | 2515 | 2578 | 2578 |
| 81 | xDFG.82 | 2527 | 2527 | 2590 | 2590 |
| 82 | xDFG.83 | 2547 | 2547 | 2610 | 2610 |
| 83 | a.l.84 | 2554 | 2554 | 2617 | 2617 |
| 84 | a.l.85 | 2573 | 2573 | 2641 | 2636 |
85 rows × 5 columns
[10]:
ref_key = "W321@3W32" # We take this one but could be any one
ref_geom = representatives[ref_key]
for key, geom in representatives.items():
if key!=ref_key:
ref_CAs, key_CAs = KLIFS_alignment.CAidxs[[ref_key, key]].values.T.astype(int)
geom.superpose(ref_geom, atom_indices=key_CAs, ref_atom_indices=ref_CAs)
Visualize residues with different behaviors in each compound
For example, residues
775@b.l.36841@c.l.74855@xDFG.81997@EGFR(doesn’t have a KLIFS label)
[12]:
colors = {key: matplotlib.colors.to_hex(col) for key, col in colors.items()}
iwd = nglview.NGLWidget()
for ii, (key, rep) in enumerate(representatives.items()):
iwd.add_trajectory(rep)
iwd.clear_representations(component=ii)
iwd.add_cartoon(color="white", component=ii)
iwd.add_licorice(color=colors[key], component=ii, selection="(775 841 855 997) and not Hydrogen", radius=.1)
iwd.add_ball_and_stick(color=colors[key], component=ii,
selection="not protein and not Hydrogen",
radius=.1,
)
iwd
References
The crystal structure of EGFR T790M/C797S with the inhibitor HCD2892 (PDB ID 7VRE)
Chen, H., Lai, M., Zhang, T., Chen, Y., Tong, L., Zhu, S., … Ding, K. (2022). Conformational Constrained 4-(1-Sulfonyl-3-indol)yl-2-phenylaminopyrimidine Derivatives as New Fourth-Generation Epidermal Growth Factor Receptor Inhibitors Targeting T790M/C797S Mutations. Journal of Medicinal Chemistry, 65(9), 6840–6858. https://doi.org/10.1021/acs.jmedchem.2c00168
EGFR kinase domain complexed with compound 20a (PDB ID 3W32)
Kawakita, Y., Seto, M., Ohashi, T., Tamura, T., Yusa, T., Miki, H., … Ishikawa, T. (2013). Design and synthesis of novel pyrimido[4,5- b ]azepine derivatives as HER2/EGFR dual inhibitors. Bioorganic & Medicinal Chemistry, 21(8), 2250–2261. https://doi.org/10.1016/j.bmc.2013.02.014
Aertgeerts, K., Skene, R., Yano, J., Sang, B. C., Zou, H., Snell, G., … Sogabe, S. (2011). Structural analysis of the mechanism of inhibition and allosteric activation of the kinase domain of HER2 protein. Journal of Biological Chemistry, 286(21), 18756–18765. https://doi.org/10.1074/jbc.M110.206193
Crystal Structure of EGFR(L858R/T790M/C797S) in complex with CH7233163 (PDB ID 6LUB)
Kashima, K., Kawauchi, H., Tanimura, H., Tachibana, Y., Chiba, T., Torizawa, T., & Sakamoto, H. (2020). CH7233163 Overcomes Osimertinib-Resistant EGFR-Del19/T790M/C797S Mutation. Molecular Cancer Therapeutics, 19(11), 2288–2297. https://doi.org/10.1158/1535-7163.MCT-20-0229